Loren Bonner
In the early months of 2021, when COVID-19 vaccines were finally available in the United States, creative community outreach programs such as mobile health units were gaining a foothold in underserved communities where COVID-19 vaccination access was low. Many of these programs were supported by savings from the 340B drug pricing program, according to Daniel Buffington, PharmD, MBA, FAPhA, associate professor at the University of South Florida Taneja College of Pharmacy.
“2020 was an unprecedented year of turbulence for the whole economy, but significantly for health care,” said Buffington during an APhA2021 Virtual session on extending care with the 340B pricing program. “There are changes and issues within the 340B program […] changes where pharmacists have played a vital role from COVID-19 testing to treatment to vaccination programs.”
He noted that the 340B program has provided “a tremendous opportunity for pharmacists and pharmacy departments.”
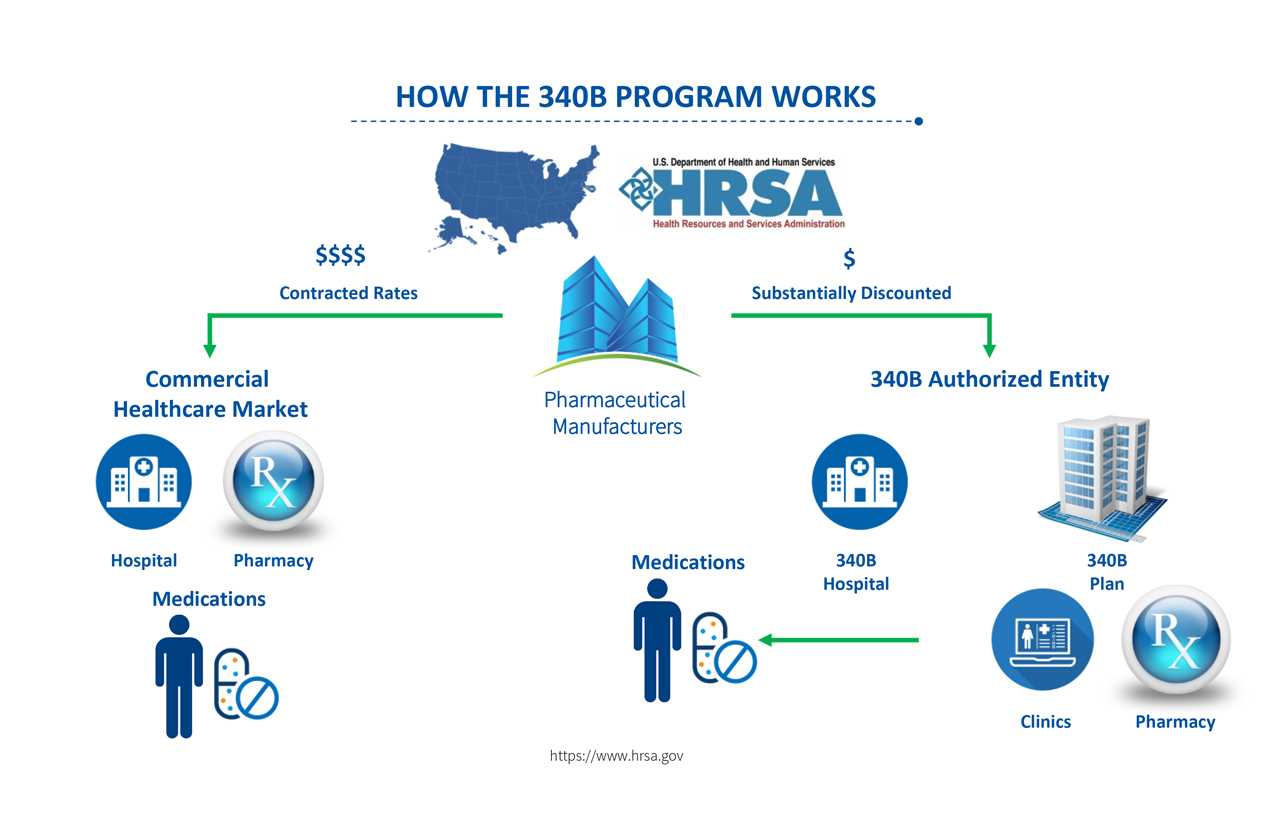
The federal 340B drug pricing program, created in 1992, requires drug manufacturers to provide as much as a 50% discount to covered entities—including federally qualified health centers (FQHCs), clinics, safety net and disproportionate-share hospitals, rural facilities, and pharmacies contracting with any of these entities.
Increasing access
The 340B program’s core objective is to increase access to outpatient medications for uninsured and vulnerable patients.
The medication savings from the program are a direct driver for expanded access to primary care, diagnostic services, and specialty services for underserved patients which addresses health care disparities, said Buffington.
According to Pam Heath, RPh, senior director of pharmacy for MCR Health, Inc., an FQHC in Florida, FQHCs are directly located in the underserved patients’ communities.
“340B funding is critical for FQHCs to provide care,” she said.
Heath said 340B entities like MCR Health, and the pharmacists who work there on the health care teams, were essential in the initial COVID-19 response and continue to be crucial.
Far from settled
In the last 10 years, Buffington said, some major changes have come about in the way the Health Resources and Services Administration (HRSA)—the entity that administers the 340B program—participates in the program.
In 2017, CMS pushed through cuts that reduced Medicare Part B reimbursements. After a legal bid to stop them failed, the cuts took effect on January 1, 2018.
In recent years, several manufacturers have opted independently to withdraw certain medications, and some of those medications have cycled back in and become part of the program, according to Buffington.
“Those disruptions when products come and products go make a real big part of where pharmacists play a vital role in tracking,” he said.
Several of the hospitals and health systems that were engaged under HRSA contracts have expressed concerns about how HRSA is managing those pharmaceutical company commitments.
Pending lawsuits from hospitals and competing federal legislation spell even more uncertainty for 340B facilities.
“This area of tension is not settled yet or resolved,” said Buffington. “There are clearly issues that need to be addressed on both sides.”