CDC releases detailed adult immunization schedule for 2024, with revisions
CDC’s adult immunization schedule for 2024, which was published in the Annals of Internal Medicine on January 12, 2024, includes updates for several vaccines, such as those for respiratory syncytial virus (RSV), meningitis, mpox, and COVID-19. The immunization schedule is based on the recommendations of CDC’s ACIP.
In October 2023, ACIP voted to approve the adult immunization schedule. Specifically, the changes from the previous iteration include the removal of the bivalent mRNA-based COVID-19 vaccines, which are no longer recommended. Current mRNA-based COVID-19 vaccines are monovalent.
Also removed from the guidance are all mentions of meningococcal serogroups A, C, W, Y polysaccharide diphtheria toxoid conjugate vaccine (Menactra), which is no longer distributed in the United States. While Menactra, or MenACWY-D, is no longer recommended, pentavalent meningococcal vaccine, or MenACWY-TT/MenB-FHbp (Penbraya), is now included.
Among other changes, the schedule has been revised to now include modified Vaccinia Ankara vaccine (Jynneos) for protection against mpox.
Additionally, clarifications have been added regarding recommendations for the measles, mumps, and rubella vaccine; hepatitis A and B vaccines; HPV vaccine; and tetanus, diptheria, and pertussis vaccines.
In an accompanying editorial to the 2024 schedule in the Annals of Internal Medicine, Scott Ratzan, MD, and other members of the Council for Quality Health Communication cited the recent CDC alert for health care providers with an “urgent need to increase immunization coverage for influenza, COVID-19, and RSV.”
In the editorial, they criticized CDC’s complex written and visual presentation of the recommendations.
“The Recommended Adult Immunization Schedule article and recent CDC alert on seasonal flu, COVID-19, and RSV vaccination shortfalls are the latest warning signs that the CDC needs to upgrade its health communication capability now,” they wrote. “Our nation’s health and security depend on it.”
The full ACIP recommendations for each vaccine are available at www.cdc.gov/vaccines/hcp/acip-recs/index.html. ■
Why are cases of syphilis soaring?

CDC has stated that the United States is seeing the highest incidence of syphilis since 1950. In its new report, the agency mapped out an astonishing comeback for a disease that had previously reached near-eradication status.
The most recent count puts the number of diagnosed new infections at more than 207,000 in 2022—a staggering 80% increase from 2018. Experts point to multiple drivers behind the trend, including a rise in substance use, which is associated with risky sexual behavior, and a dearth of sexual health clinics.
The epidemic is also affecting every age group, including newborn babies, and is having an especially pronounced impact on Black Americans and people of Native American/Alaskan Native heritage.
Without proper care, the virus can damage organs; lead to loss of eyesight and hearing; cause paralysis; and result in miscarriage, stillbirth, or developmental delays in babies.
The Biden administration has unfurled initiatives designed to address the high rate of infection, including formation of a national task force, temporary importation, an alternative syphilis intervention because Bicillin L-A is in short supply in the United States, and ongoing development of a simple test for use in clinics. ■
FDA adds boxed warning to denosumab for patients with advanced CKD
FDA added a boxed warning to the osteoporosis drug denosumab (Prolia). The agency’s review of the drug indicated risks that warrant new prescribing information. Patients with chronic kidney disease (CKD)—especially those on dialysis or those with a concomitant diagnosis of mineral and bone disorder could develop severe hypocalcemia, or very low levels of calcium in the blood.
FDA is changing the label for denosumab to include a boxed warning to reflect the risk of hospitalization and even death for this group of patients.
Added information will provide advice to both patients and providers on strategies to curtail the risk of harm, including a recommendation to evaluate kidney function in patients before prescribing denosumab, emphasis on the importance of sufficient calcium and vitamin D intake for current users, and a recommendation for frequent monitoring of blood calcium levels for patients with advanced CKD. ■
Early tecovirimat treatment for mpox disease may benefit people with HIV
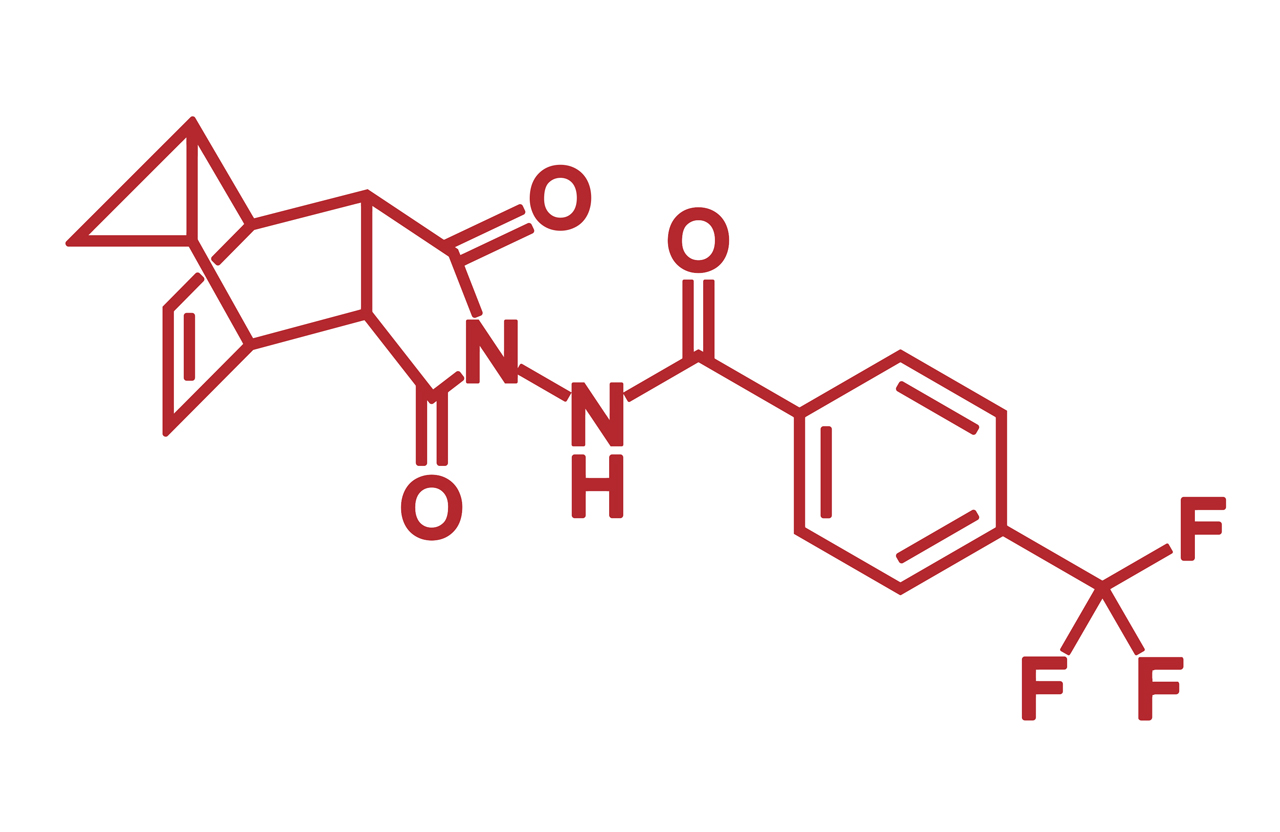
Early intervention with tecovirimat may stop an mpox infection from advancing to the stages of severe disease in people living with HIV (PWH), new research in JAMA Internal Medicine suggests.
Investigators followed a sample of PWH who received care for mpox at four Atlanta hospitals between June and Oc-tober 2022. Two propensity-matched cohorts were formed: one including 56 patients who received tecovirimat therapy within 7 days of mpox symptom onset, and the other consisting of 56 patients who did not receive tecovirimat for at least 7 days after symptom onset or never received it at all.
Mpox disease progression was observed in 5.4% of patients in the early treatment group compared with 26.8% in the late- or no-treatment group. ■
FIP represents, advocates for pharmacy at WHO

The International Pharmaceutical Federation (FIP) represented pharmacy at a January 2024 session of the WHO Executive Board in Geneva, Switzerland. Some of the main themes on the agenda included
universal health coverage, health emergencies, and health and well-being.
On the topic of antimicrobial resistance, for instance, FIP reminded members that as frontline health care professionals, pharmacists play a crucial role in combating antimicrobial resistance.
WHO’s work in health emergencies was another widely discussed topic. The World Health Professions Alliance, which includes FIP, gathered data during the COVID-19 pandemic and because of those efforts, a new clause was placed in the WHO’s Pandemic Accord, requiring parties to protect the safety of health professionals in emergencies, according to Paul Sinclair, president of FIP. This includes ensuring priority access to personal protective equipment.
FIP is the global body for pharmacy, pharmaceutical sciences, and pharmaceutical education. APhA is a member organization of FIP. ■
Study finds possible risk of birth defects with first trimester use of methadone versus buprenorphine

Both buprenorphine and methadone treat OUD, but there is limited understanding of how in-utero exposure affects infants. Researchers of a new study published in JAMA Internal Medicine used Medicaid data to gauge the risk of congenital malformations in neonates whose mothers received buprenorphine or methadone while carrying them.
After examining Medicaid data from more than 13,000 pregnancies during 2000 to 2018, outcomes data showed that birth defects were more prevalent overall with first trimester exposure to methadone than with similar exposure to buprenorphine. In 9,514 cases, pregnant parents received buprenorphine during the first 90 days of gestation. In 3,846 cases, they received methadone.
The relative risk reduction with buprenorphine was 18%, the equivalent of one fewer event per 100 patients compared with methadone. Similar patterns emerged when investigators looked specifically at birth defects linked with opioid use, the only exception being GI malformations, which were more likely with fetal exposure to buprenorphine.
They noted that risk of birth defects is just one factor to consider when choosing an intervention for OUD in pregnant women. Access to treatment, previous treatment success, and the odds of retention in treatment should also inform the decision, they added. ■